Zundel ion catalyst hypothesis
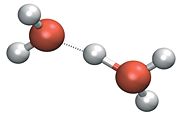
Hydronium ions in the H5O2+ form are now referred to as Zundel cations, and are regarded as an hydronium ion with an additional water molecule attached.
Aim
There is epidemiological support for a connection between water and cancer, and my thoughts are that it's profitable to consider a way in which Zundel and Eigen ions might be involved in this process.
So this hypothesis aims to show that there is a chemical process by which glycine in DNA might be converted to valine as a result of Zundel or Eigen ions being involved.
Zundel ions
As shown above, Zundel ions are hydronium ions with an extra water molecule attached. The bond between an hydronium and water is stronger than that between water molecules, around 4.3 kcal./mole compared to 2.5 kcal./mole. (Omer Markovitch and Noam Agmon. Structure and energetics of the hydronium hydration shells. Journal of Physical Chemistry A, 111 :2253-2256, 2007 (Letter)):
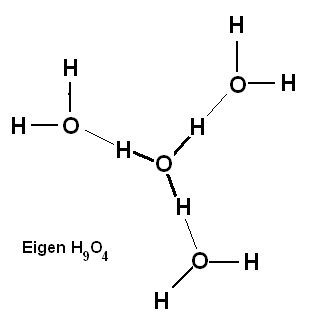
I will assume that the currently accepted arrangement of the Zundel ion can be seen as an hydronium ion with a water molecule attached, the hydronium having strong internal bonds and the water being weakly attached and that this arrangement probably extends to more than one water molecule to form the Eigen ion with 3 water molecules weakly attached:
Methanol
In this suggested system the Zundel ion interacts with methanol where the hydronium ion is detached from the water molecule and attached to methanol:
Why this hypothesis? As a way forward in my research, I'm putting forward an idea which might plausibly explain how conductive water pipe affects cancer through ionisation. It is not to prove: it's to give direction to my research until proved, disproved or made redundant by a better idea. Until then, and acknowledging it's shortcomings, this is my best hunch.
It would appear, however, that there are different evaluations of this bond strength with some accounts giving it a higher value, up to around 34kcal./mole. (Ojamae, L.; Shavitt, I.; Singer, S. J.; Int. J. Quantum Chem., Quantum Chem. Symp. 1995, 29, 657.). I will be taking that the 4.3 kcal./mole as correct, as it is by test rather than calculation.
OH3+•OH2 + H3C•OH ===> H3C•OH•H3O + H2O
zundel ion plus methanol gives protonated methanol plus water
Methanol would not normally be expected to do this: quite the contrary in fact. My hunch here is that Otto Warburg showed that cancer can be reliably caused by anaerobic conditions, and methanol is produced by many anaerobic bacteria, so this may relate. The glitch with following this path is that the hydronium tends to move from methanol to water in solution, so the equation would appear to be the wrong way around.
Source: Wikipedia
Conclusion
© Stephen G. Butcher (Posted 27/07/08)
(Revised 04/08/08)
If, however, we say that methanol is not in solution in a cell then we could also say that the hydronium might not migrate to water molecules. I base the arguement for 'not in solution' on the way in which chlorine is used in water to kill bacteria by knocking down the cell wall: death by drowning. So I will assume the cell in which this process might occur is not drowned (although it may be damaged). Ken-ichiro Suhara, Asuka Fujii and Naohiko Mikami (under construction) found that when the hydronium ion is attached to between 6 and 9 protonated methanols there is no evidence of proton migration to water:
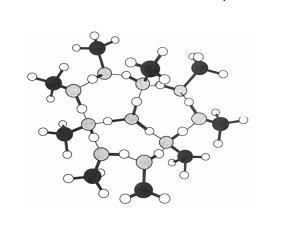
This picture is of the 9 protonated methanol structure, but the arrangement for 6 rather than 9 is self explanatory.
The weak hydrogen to oxygen bonds between the central hydronium and the methanol oxygens are indicated by the thin lines, as are the weak H-O bonds between adjacent methanols.
I make no guarantees about the above equation, but if we assume that there is a means by which the hydronium becomes attached to the methanol then we can look further at the effect this might have on DNA.
Glycine
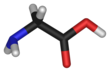
Glycine is the simplest form of amino acid, into which there can theoretically be added CH2 groups. To change this to a valine (for accelerated cell growth) we need to add CH2 groups plus a CH3 terminal group, after breaking off an hydrogen (indicated) to allow a carbon to carbon (C-C) bond.
If we look at the first CH2 to be added it might look something like:
H2N•CH2•COOH + H3C•OH•H3O ===> H2N•CH(CH3)•COOH + H3O•OH2
The bond energies would be:
C-H broken = +98 kcal./mole
C-O broken = +78 kcal./mole
C-C formed = - 80 kcal./mole
O-H formed = -110 kcal./mole
Total = 14 kcal./mole released
So this process of breaking off an hydrogen and substituting a CH3 group three times would result in a valine. The hydronium core groups methanols in three so it may be possible that this relates.
Gylcine (Source: Wikipedia)
Valine (Source: Wikipedia)
So in a sense the CH3 group is exchanged for an hydrogen, and the hydronium core continues on its way as a facilitator of this exchange process.
If so, then the hydronium is a catalyst rather than being irreversibly changed and, presumably, the more hydroniums there are the faster this process can proceed.
This may raise more questions than it answers. If pH of reticulated water is controlled then so too would be the supply of hydronium ions. But if there is a process by which the bond energy within the hydronium is increased then perhaps this would make the ion more permanent.
One line of enquiry might be to see if there is a difference in intramolecular hydronium bonds between countries of differing cancer rates. For instance, would the O-H bond in hydronium be less in Israel than in the United States and, if so, by how much?
Suhara et al
H2OH+(4.3)OH2 compared to HOH(2.5)OH2